It is well-known that batteries are vital to building an electric and fossil-free society. The technical advancements in the past decade have empowered us to work toward a more sustainable energy future – and the process began over 300 years ago.
1700's: Animal Electricity and The Battery Principle
In 1780, Italian biologist Luigi Galvani marked the first discovery of the battery principle. The observation came during a frog dissection when he noticed its legs twitching when two different types of metal, copper and iron, touched it. This became known as "animal electricity."
In 1792, Italian physicist, Alessandro Volta, turned his focus to the experiments conducted by Galvani. However, Volta's attention was drawn to the metallic elements rather than the biological aspects of Galvani's work. Through this, Volta realized that the frog was the conductor of these two metals. In his quest for further understanding, Volta conducted experiments by placing the metals on his tongue. He later continued his experiments with a sole focus on the metals leading him to a crucial discovery: living tissue was not necessary to generate an electric current.
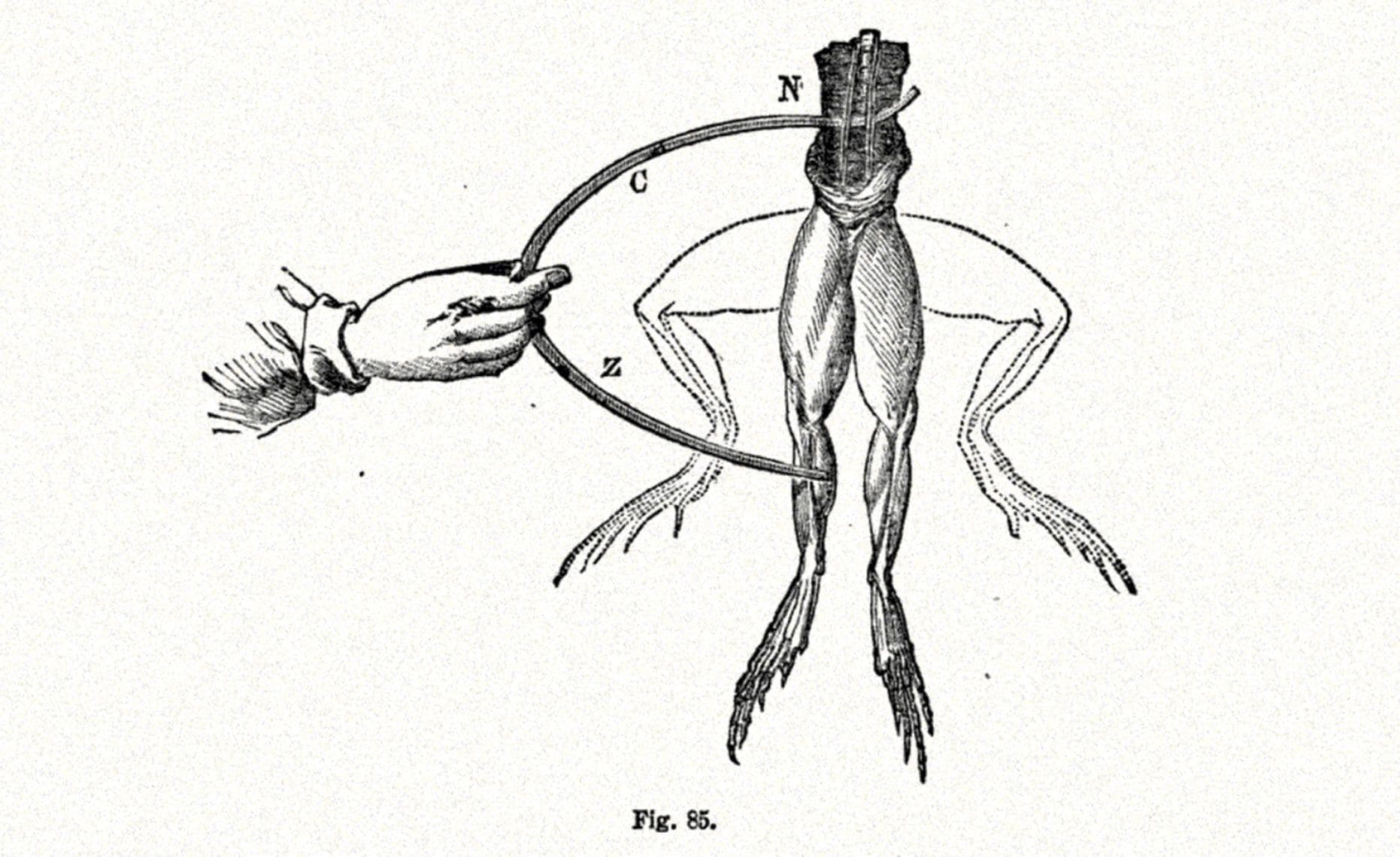
Galvani's Frog Experiment
1800's: The Voltaic Pile and Battery Storage
Building upon this insight, Alessandro Volta introduced his groundbreaking invention in 1800, the Voltaic Pile. This battery consisted of alternating discs of metals, such as copper and zinc, separated by a cloth soaked in salt water. Through this, Volta successfully demonstrated electricity generation, marking a milestone in future advancements in electric technology. Within weeks of the discovery, English scientists William Nicholson and Anthony Carlisle used the experiment to discover electrolysis, creating the scientific field we now know as electrochemistry.
By 1887, the dry battery concept evolved when a German scientist, Carl Gassner, patented the Dry Cell Battery Concept – a battery with no risk of water spillage, derived from Georges Leclanché's dry battery (1868) using ammonium chloride, which caused said spillage. This cell used zinc as the container and negative electrode, while carbon and manganese were the positive electrodes. Ammonium chloride was used as the electrolyte, and paper was used as a separator between the electrolyte and zinc anode. The experiment was improved by adding zinc chloride to the electrolyte and reducing the corrosion for a longer-lasting cell.
Subsequently, Swedish engineer Waldemar Jungner observed that these batteries were experiencing problems. They were too big and lacked the power to supply equipment with enough energy to work long hours. In 1899, he developed a portable Nickel-Cadmium battery. The cell uses nickel as a positive anode, cadmium as a negative, and an alkaline solution as the electrolyte. The battery could charge faster, last longer, and was mostly silent. Unfortunately, the battery could not compete then because the materials were too expensive, but it is the foundation for today's battery storage.
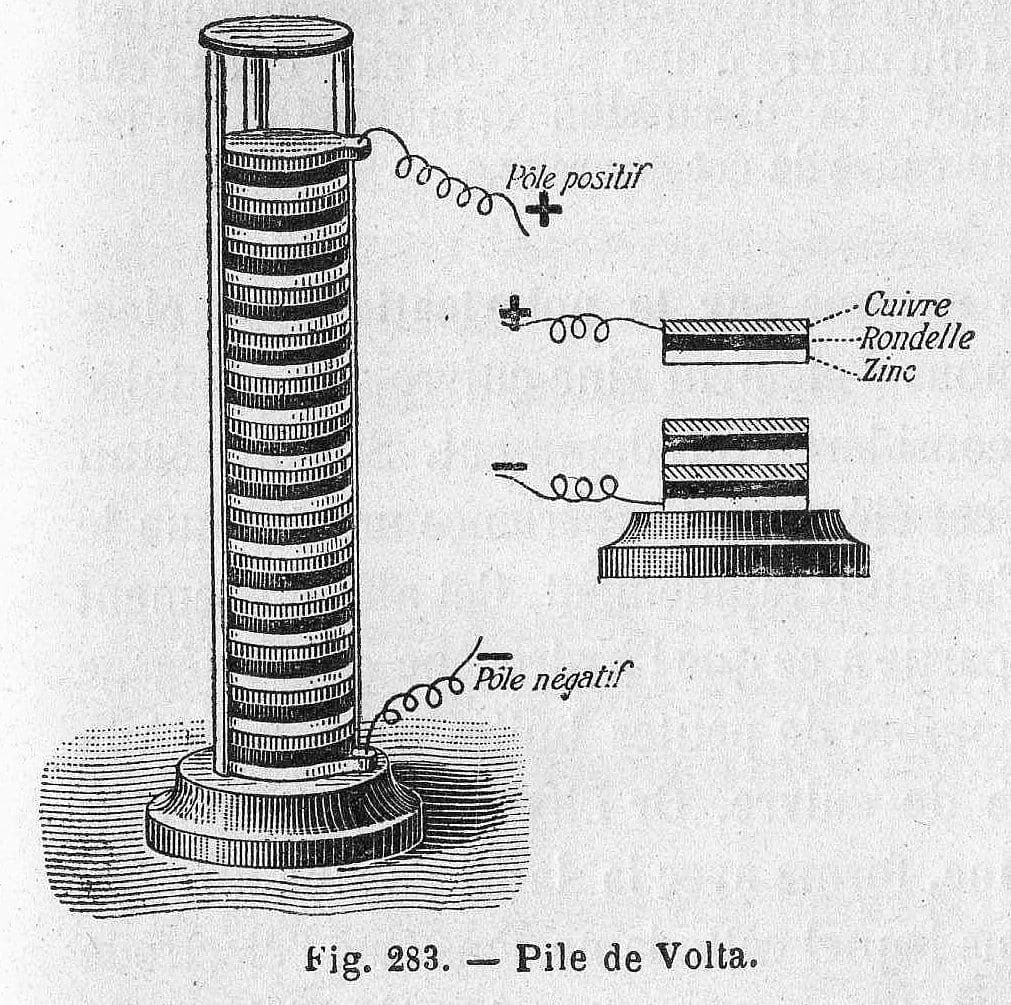
Volta's Voltaic Pile
1900's – Today: Nickel-Iron Storage and Lithium-Ion Batteries
From these early experimentations, battery development rapidly increased with Thomas Edison's invention of the nickel-iron storage battery in 1900. By 1954, the first full metal jacket dry battery was released in Japan, leading to the launch of mercury battery production in 1955.
In 1980, American physicist John B. Goodenough invented the Li-ion battery, in which lithium migrated through the battery from one electrode to another, creating the base for the lithium-ion battery. In this battery, lithium is combined with a "transition metal," such as cobalt, nickel, manganese, or iron, and oxygen to form the cathode. When voltage is applied, the positive lithium ion from the cathode migrates to the graphite anode and becomes lithium metal. 1990 Goodenough made another leap in battery technology by introducing a stable lithium-ion cathode based on lithium iron and phosphate, propelling innovation, research, and development of modern-day batteries and energy storage.
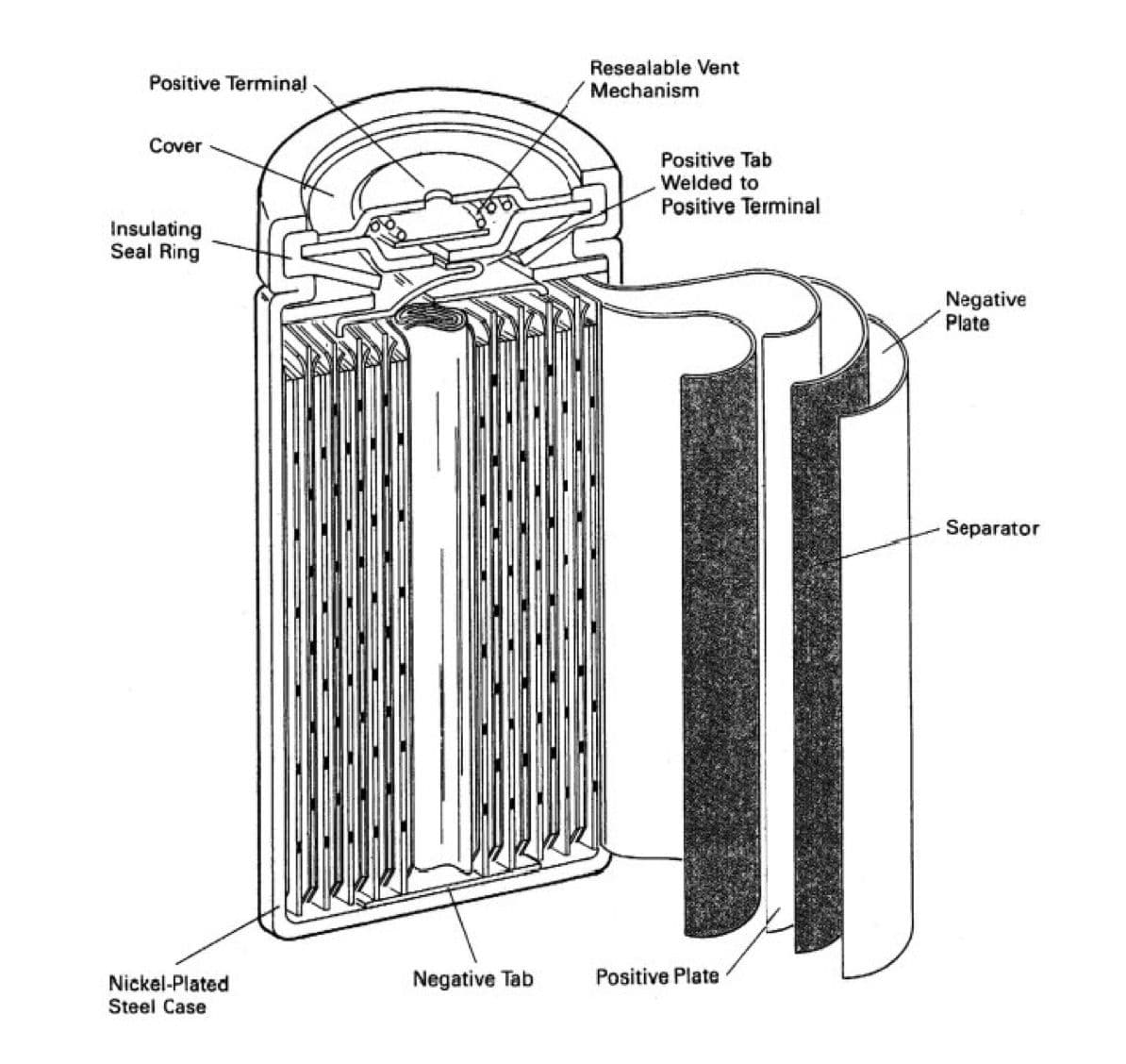
Jungner's Portable Battery




